Laminated Rubber Stoppers



Daikyo Flurotec® lamination, originally developed by DAIKYO, is used in all of DAIKYO's rubber stoppers and is licensed to West Pharmaceutical Services,Inc.
The barrier effect of this Daikyo Flurotec® fluoropolymer film inhibits the interaction between the rubber stopper and the drug.
It can also contribute to the reduction of the total cost of drug formulation production thanks to its excellent durability and quality retention.
Main applications
- Serum drugs
- Powder drugs
- Lyophilized drugs
- Infusion
formulations - Low molecule
formulations - Gene therapy
- Animal Health
- Vaccine
- Generic
- Biological drugs
- Bio Similar
Characteristics of elastomers
-
Low absorbency of
proteins and others -
Chemical resistance/
Low extractables/
Leachables -
Low moisture
absorption -
Chemical
inert -
Low gas permeability
-
Non-adhesive
Advantages of Introduction
Laminated based on the usage
Daikyo Flurotec®, laminated on the necessary part such as drug contact area and top part for lyophilization. The interaction between the rubber and the drug is reduced to reduce metal ions and particulates, while at the same time ensuring a high degree of sealing with the vial and avoid the lyo stoppers sticking to the lyo chamber. In addition, RB2 coating is also combined to prevent docking between rubber stoppers and to ensure smooth machine transportability.


Unique washing process and
100% vision inspection
Our unique 3 step washing process in ISO7 clean room achieves 3 log reduction of Endotoxin and the water for washing such as RO water and WFI are produced in-house.
All products are vision inspected by vision inspection machine with 0.05㎟ inspection spec which enables to reduce stoppers with defects and improve the yield rate at the pharmaceutical production sites.
*There are some exceptions; D SIGMA products are 0.01㎟ or higher.

Two main DAIKYO formulations
Original and high quality rubber materials are manufactured at our own plants in Japan, with optimal compounding of original materials that conforms JP/EP/USP/ChP. We can recommend the formulations based on the usages.

DAIKYO elastomer products portfolio
DAIKYO have various stoppers for small vials to large vials.
-
DAIKYO RSV®
Ready to sterilize validated
-
DAIKYO RUV®
Ready to use validated
-
DAIKYO D SIGMA
Pinacle quality of DAIKYO stoppers
DAIKYO RSV®
Washing validated
products
DAIKYO dramatically reduces adhered foreign matters by unique washing process.
-
Washing validated
products -
100% camera inspected with
0.05㎟ vision inspection spec -
Manufactured in ISO7
Clean room -
Filed in DMF(US/CANADA/CHINA)
-
Production plants are certified for ISO 9001/15378
-
Portedbag applicable for primary bag (GLC190/SSB110)
RSV Endotoxin/Bioburden/Particle test
| Test items | Spec | |
|---|---|---|
| Endotoxin test(DAIKYO original test method) | <0.05 EU/mL | |
| Bioburden test (DAIKYO original test method) |
Bioburden | ≤50CFU |
| Particle test (DAIKYO original test method) |
≥2µm | ≤200Pieces /10mL |
| ≥5µm | ≤20Pieces /10mL | |
| ≥10µm | ≤5Pieces /10mL | |
| ≥20µm | ≤3Pieces /10mL | |
| ≥30µm | ≤0Pieces /10mL | |
Batch size
| 13mm Serum stoppers |
20mm Serum stopper |
13mm Lyo stoppers |
20mm Lyo stoppers |
||
|---|---|---|---|---|---|
| Sterilizable bag |
Pieces per bag | 7,500 | 2,500 | 5,000 | 1,750 |
| Pieces per carton | 15,000 | 5,000 | 10,000 | 3,500 | |
| Pieces per batch | 480,000 | 200,000 | 320,000 | 140,000 | |
| GLC190 Port Bag |
Pieces per bag | 13,000* | 4,000 | 10,000 | 3,000 |
| Pieces per carton | 13,000* | 4,000 | 10,000 | 3,000 | |
| Pieces per batch | 468,000* | 144,000 | 320,000 | 108,000 | |
| SSB110 Port Bag |
Pieces per bag | 10,000* | 3,000 | 8,000* | 2,000 |
| Pieces per carton | 10,000* | 3,000 | 8,000* | 2,000 | |
| Pieces per batch | 360,000* | 108,000 | 288,000* | 72,000 | |
*Please contact us for the detailed information.
DAIKYO RUV®
Sterilization
validated
DAIKYO RUV® enables customers to use the stoppers without further processes and reduces total cost of ownership. DAIKYO RUV® is based on DAIKYO RSV® which the foreign matter is reduced, is further autoclave sterilized by DAIKYO.
-
Autoclave sterilization
validated -
100 % camera inspected with
0.05㎟ vision inspection spec -
Manufacured in ISO 7 clean
room, Sterilized in ISO 5
clean room -
Filed in DMF(US/CANADA/CHINA)
-
Production plants are certified for ISO 9001/15378
-
Ported bag applicable for primary bag (GLC190/SSB110)
RUV Endotoxin/Bioburden/Particle test
| Test items | Spec | |
|---|---|---|
| Endotoxin test (DAIKYO original test method) | <0.05 EU/mL | |
| Bioburden test(DAIKYO original test method) | ≤50 CFU/250mL | |
| Particle test (DAIKYO original test method) |
≥2µm | ≤200Pieces /10mL |
| ≥5µm | ≤20Pieces /10mL | |
| ≥10µm | ≤5Pieces /10mL | |
| ≥20µm | ≤3Pieces /10mL | |
| ≥30µm | ≤0Pieces /10mL | |
*Test sample is RSV sample.
Batch size
| 13mm Serum stoppers |
20mm Serum stoppers |
13mm Lyo stoppers |
20mm Lyo stoppers |
||
|---|---|---|---|---|---|
| Sterilizable bag |
Pieces per bag | 7,500 | 2,500 | 5,000 | 1,750 |
| Pieces per carton | 7,500 | 2,500 | 5,000 | 1,750 | |
| Pieces per batch | 480,000 | 200,000 | 320,000* | 140,000* | |
| GLC190 Ported Bag |
Pieces per bag | 13,000* | 4,000 | 10,000 | 3,000 |
| Pieces per carton | 13,000* | 4,000 | 10,000 | 3,000 | |
| Pieces per batch | 468,000* | 144,000 | 320,000* | 108,000* | |
| SSB110 Ported Bag |
Pieces per bag | 10,000* | 3,000 | 8,000* | 2,000 |
| Pieces per carton | 10,000* | 3,000 | 8,000* | 2,000 | |
| Pieces per batch | 360,000* | 108,000 | 288,000* | 72,000* | |
*Please contact us for the detailed information.
DAIKYO D SIGMA
Washed
product
Sterilized
product
A new brand of DAIKYO elastomer products developed to reduce container-derived foreign matter and defects more than ever before. Among DAIKYO RSV® (Ready to sterilize validated)/DAIKYO RUV® (Ready to Use validated) elastomers, which boast a high share of the pharmaceutical and medical rubber stopper market, only those that have passed more stringent inspections, including foreign matter inspection and dimensions, are shipped as this D SIGMA. D SIGMA's strict standards can contribute to further yield improvement of drug manufacturing, leading to lower total cost of ownership.
-
Choice of RSV or RUV
-
100% camera inspection with
0.01㎟ vision inspection spec -
100% Dimension measured
during Vision inspection
process -
Particle test based on
ISO 8871-3 -
Tighter AQL
-
Available in Ported bag(GLC190/SSB110)
Particle test for D SIGMA(Same as RSV/RUV)
Membrane filtration method(ISO8871-3)
| Particle Diameter | D SIGMA RSV / RUV |
|---|---|
| ≥ 25.0µm - 50.0µm | ≤ 10.0Pieces / 10c㎡ |
| ≥ 50.0µm - 100.0µm | ≤ 1.0Pieces / 10c㎡ |
| ≥ 100.0µm | ≤ 0.2Pieces / 10c㎡ |
Light Obscuration method(DAIKYO original test method)
| Particle Diameter | D SIGMA RSV / RUV |
|---|---|
| ≥ 2µm | ≤ 200Pieces / 10mL |
| ≥ 5µm | ≤ 20Pieces / 10mL |
| ≥ 10µm | ≤ 5Pieces / 10mL |
| ≥ 20µm | ≤ 3Pieces / 10mL |
| ≥ 30µm | ≤ 0Pieces / 10mL |
Tighter AQL
| Level | AQL(%) | Number of samples | Ac , Re | ||
|---|---|---|---|---|---|
| Critical |
Standard/RSV products Lot size: 150,001-500,000 0.015 |
D SIGMA products
Lot size: 150,001-500,000 0.015 |
800 |
Standard/RSV products Lot size: 150,001-500,000 0, 1 |
D SIGMA products
Lot size: 150,001-500,000 0, 1 |
| Major | 0.25 | 0.10 | 800 | 5, 6 | 2, 3 |
| Minor | 1.00 | 0.25 | 800 | 14, 15 | 5, 6 |
Final sampling appearance inspection is performed based on ANSI/ASQZ1.4-2003(R2018) level II one-time sampling method inspection.
Size and batch size is same as RSV/RUV
How we package our RUV(Sterilized) products
To prevent the contamination during transportation, sterilization validated product DAIKYO RUV® as well as D SIGMA RUV have two packaging configurations depending on the stoppers.
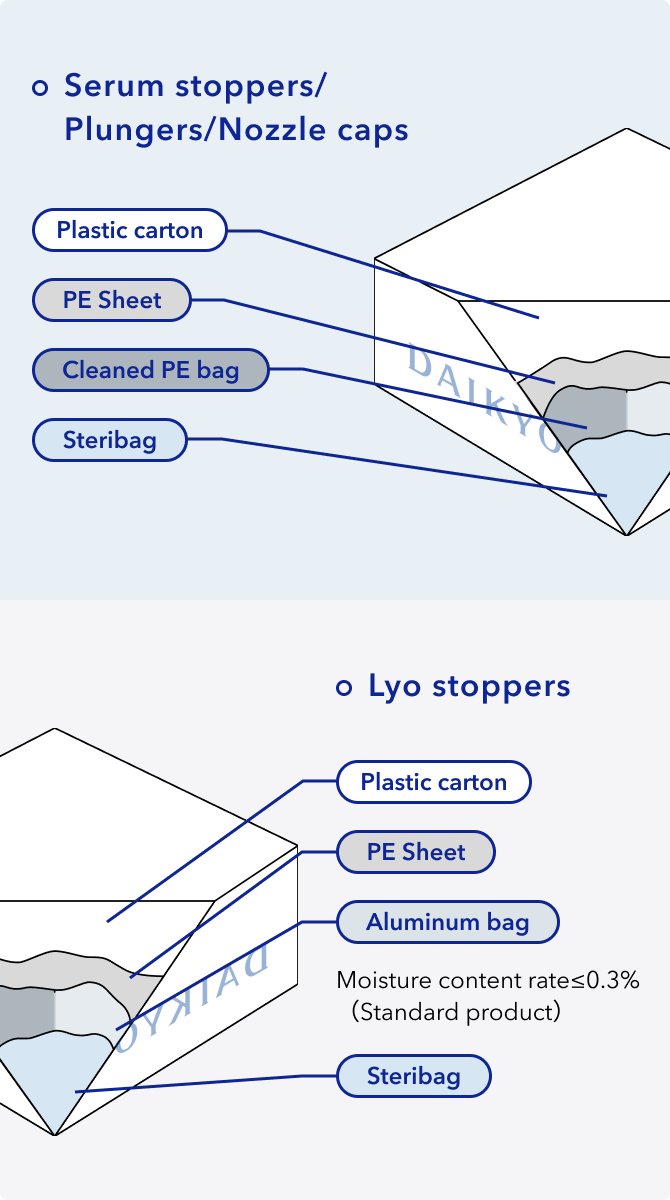
Portedbag available
DAIKYO RSV®/RUV®,D SIGMA available in portedbag
Based on the customer request, we will try to increase the packaging configuration.
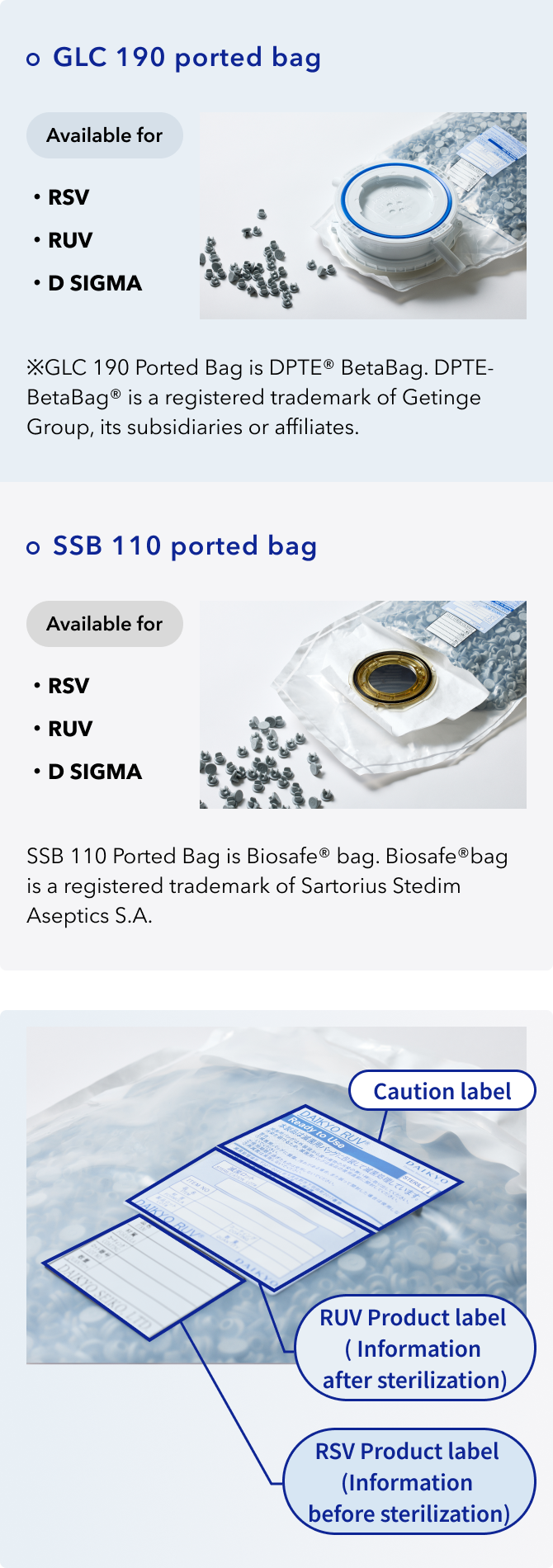
Stopper information based on usage
Below are examples. For detailed information, please contact us from the inquiry.
Stoppers for ISO Glass vials
| Serum stoppers | Lyo stoppers | ||
|---|---|---|---|
| ISO 13mm | NBB | S2-F45-2 |
V2-F451W (Stoppers with thicker puncture area) V2-F10-2W |
| EBB/ABB | S2-F451 | ||
| ISO 20mm | NBB | S10-F57 |
V10-F451W (Stoppers with thicker puncture area) V10-F597-2W |
| EBB/ABB | S10-F451 | ||
EBB:European blow back
ABB:American blow back
NBB:Non blow back
Stoppers recommended for CSTD
| Serum stoppers | Lyo stoppers | ||
|---|---|---|---|
| CSTD | 13mm | S2-F45-5 |
V2-F451W (Stoppers with thicker puncture area) V2-F10-2W |
| 20mm | S10-F57 | V10-F597-2W | |
Please contact us for elastomer formulation with less moisture content rate.
Stoppers recommended for CZ Vials
| Serum stoppers | Lyo stoppers | |
|---|---|---|
| Conical 0.5 | S2-F45-2 | |
| Vial 2A13-3 | S2-F45-5 for CSTD S2-F45-2(Stoppers with thicker puncture area) | V2-F451W(Stoppers with thicker puncture area) V2-F10-2W |
| Vial 10A20-2 Vial 50A20-2 |
S10-F57 | V10-F451W(Stoppers with thicker puncture area) V10-F597-2W |
| Vial 100A32-3 | L200-F3-2 L100-F7-2(plug diameter is larger) | V100-F8W |



